BACKGROUND: Anemia, neutropenia, and thrombocytopenia are expected complications of autologous hematopoietic cell transplant (AHCT) for multiple myeloma (MM). However, prolonged cytopenias may predispose patients to other infectious, cardiovascular, and/or hematological toxicities. Various factors have been implicated in the length of post-AHCT cytopenias including stem cell dose, marrow microenvironment, and clonal hematopoiesis. We hypothesize that the length of post-transplant anemia, neutropenia, and thrombocytopenia may negatively impact hospital length-of-stay (LOS) and in-hospital complications.
METHODS: This is a single-center observational study of MM patients who underwent AHCT. Patients were part of a larger cohort with detailed single nucleotide polymorphism (SNP) array cytogenetic data. Exclusion criteria were concurrent amyloidosis and tandem transplant. Demographic data, comorbidities, cytogenetic features (presence of TP53 deletion, and translocation status of MMSET, CCDN3, CCDN1, MAF, and MAFB), therapy line, peri-transplant laboratory values, and clinical outcome were collected retrospectively. LOS was calculated from transplant day -2 to hospital discharge. Predictive factors for LOS were calculated with multiple linear regression. Multiple logistic regression was then used to calculate associating factors for in-hospital complications. Grade III and IV post-transplant cardiovascular, infectious, and hematological complications were collected following the Common Terminology Criteria for Adverse Events (CTCAE). Post-transplant anemia was defined as sustained hemoglobin (Hb) <8 g/dL despite transfusions. The cutoffs for neutropenia and thrombocytopenia were absolute neutrophil count (ANC) <1.5 K/uL, and platelet (PLT) count <50 K/uL, respectively.
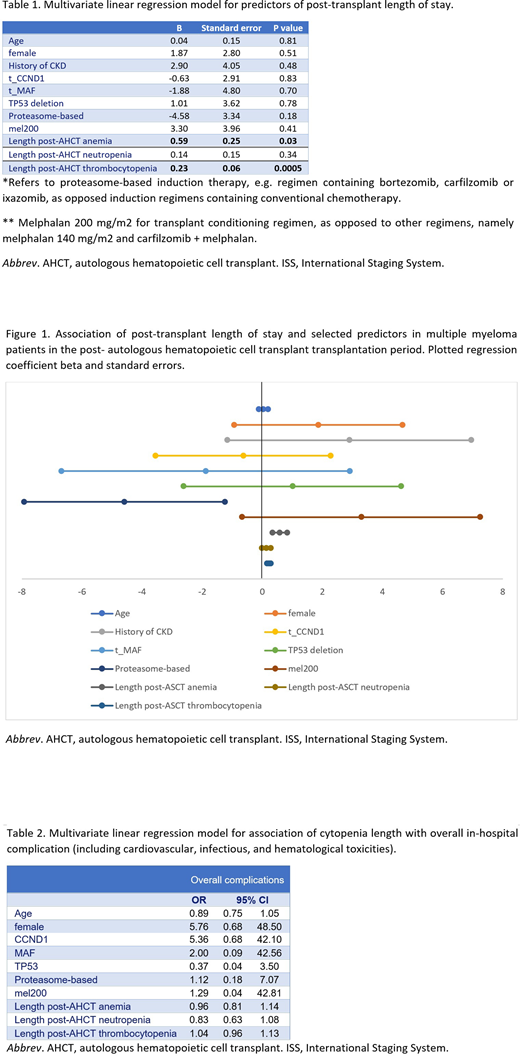
RESULTS: 158 AHCT cases of MM patients were identified. 95 patients received AHCT as frontline treatment, 35 patients were transplanted in the salvage setting, and 14 patients received both frontline and subsequent-line transplant. The most commonly used conditioning regimen was melphalan 200 mg/m2 in 123 cases, followed by melphalan 140 mg/m2 in 23 patients. 50.6% (80/158) developed grade III anemia with a median onset of 9 days after transplant (IQR 5-11 days) and median length of 2 days (IQR 1-6 days). Neutropenia had higher incidence of 96.8% (153/158) with an earlier onset of 5 days (IQR 5-6 days) and median length of 5 days (IQR 2-7 days). Grade III thrombocytopenia occurred in 98.1% (155/158), with median onset of 7 days (IQR 5-7 days) and a median duration of 8 days (IQR 6-10 days). When comparing patients who received frontline transplant vs subsequent line transplants, no statistical significance was observed between onset or length of cytopenias (p=0.40). Median LOS was 16 days (IQR 15-19 days). The most frequent post-AHCT complications in our cohort were neutropenic fever (N=27), followed by engraftment syndrome (N=12), pneumonia (N=4), urinary tract infection (N=2), cellulitis (N=2), and bacteremia (N=1). Cardiovascular events were uncommon (N=3) and included pericarditis, new onset atrial fibrillation, and new onset supraventricular tachycardia. Pulmonary embolism (N=1) and deep vein thrombosis (N=3) were recorded. No major bleeding was observed. Longer LOS was independently associated with post-AHCT anemia (p=0.03) and thrombocytopenia (p=0.0005). Notably, LOS and in-hospital complication rates were not significantly associated with demographic data, pre-existing comorbidities, pre-transplant cytopenias, or cytogenetic abnormalities.
CONCLUSION: In our cohort, longer duration of post-transplant anemia and thrombocytopenia were statistically associated with longer hospital LOS, but not with post-transplant complications. Prospective validation in an independent cohort is warranted.
Landgren:Karyopharma: Research Funding; Binding Site: Consultancy, Honoraria; Takeda: Other: Independent Data Monitoring Committees for clinical trials, Research Funding; Janssen: Consultancy, Honoraria, Other: Independent Data Monitoring Committees for clinical trials, Research Funding; Merck: Other; Seattle Genetics: Research Funding; Celgene: Consultancy, Honoraria, Research Funding; Adaptive: Consultancy, Honoraria; Juno: Consultancy, Honoraria; Amgen: Consultancy, Honoraria, Research Funding; Celgene: Consultancy, Honoraria, Research Funding; Cellectis: Consultancy, Honoraria; Juno: Consultancy, Honoraria; Glenmark: Consultancy, Honoraria, Research Funding; Seattle Genetics: Research Funding; Pfizer: Consultancy, Honoraria; Merck: Other; Karyopharma: Research Funding; Binding Site: Consultancy, Honoraria; BMS: Consultancy, Honoraria; Cellectis: Consultancy, Honoraria; BMS: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria; Janssen: Consultancy, Honoraria, Other: Independent Data Monitoring Committees for clinical trials, Research Funding; Takeda: Other: Independent Data Monitoring Committees for clinical trials, Research Funding; Glenmark: Consultancy, Honoraria, Research Funding. Chung:Genentech: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal